Abstract
Background: B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most common pediatric malignancy. Using minimal residual disease (MRD) and genetic subtypes, risk stratification algorithms have considerably improved clinical outcomes. However, despite being in a better prognostic group, a non-negligible number of relapses occur, and prognostic markers for these patients are required for more effective treatment strategies.
RNA sequencing (RNA-seq) is a useful tool for detecting patient-specific immunoglobulin heavy chain (IGH) junctional sequences (Li et al., Leukemia. 2020), which can be informative for evaluating the nature of leukemic clones. Here, we analyzed IGH clonality of pediatric BCP-ALL using RNA-seq data and revealed its validity for evaluating disease characteristics and impact on prognosis.
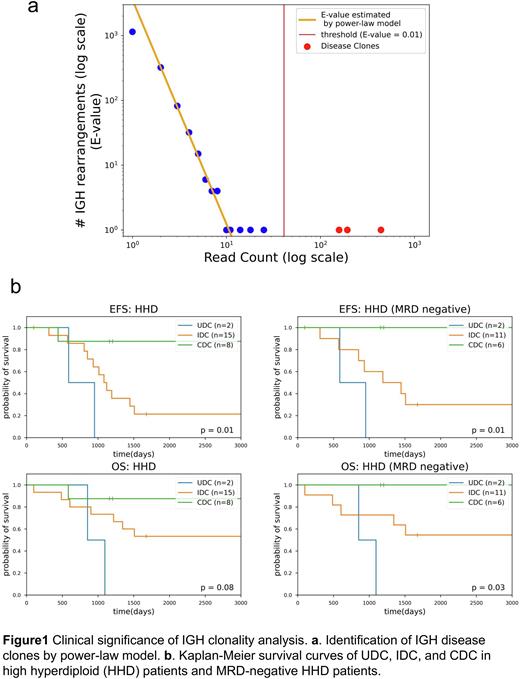
Methods: We analyzed RNA-seq data of 136 pediatric BCP-ALL samples at diagnosis and 46 samples at relapse from the TARGET ALL Expansion Phase 2 cohort. Using MiXCR (Bolotin et al., Nat Methods 2015) and Vidjil (Duez et al., PLoS One 2016), we detected IGH complete VDJ and incomplete DJ rearrangements. Then, we estimated the expected number of IGH rearrangements corresponding to each read count (E-value) by power-law model. Finally, we identified leukemic "disease clones” with E-values less than 0.01 (Fig. 1a).
Results: First, we detected a total of 55,518 IGH clones, of which 48,555 were complete rearrangements and 6,963 were incomplete. In total, 351 disease clones (median 2, range 0-8 per sample) were identified by power-law model: 193 clones were complete rearrangements, and 158 clones were incomplete. Interestingly, the numbers and compositions (complete or incomplete rearrangements) of disease clones varied significantly between samples. Thus, we defined three clonality patterns and classified each sample based on identified disease clones: "Undetectable disease clones (UDC)” if the sample had no disease clone (n=18); "Incomplete Disease Clones (IDC)” if there were one or more disease clones with incomplete rearrangements (n=72); and "Complete Disease Clones (CDC)” if all disease clones of that sample have complete rearrangements (n=46). Gene Set Enrichment Analysis (GSEA) indicated a strong enrichment for hematopoietic stem cell (HSC) and multipotent progenitor (MPP) signatures in UDC and pro-B cell signatures in IDC. Similarly, CIBERSORTx (Newman et al., Nat Biotechnol 2019) showed higher proportions of HSCs, MPPs, and pre-pro B cells in UDC and pro-B cells in IDC. Furthermore, there was a significant enrichment in pathways promoting cell cycle progression in IDC. Of note, in high hyperdiploid (HHD) cases, which are typically associated with a favorable prognosis, event-free survival (EFS) was significantly lower in UDC and IDC (p=0.01) (Fig. 1b). Moreover, even in MRD-negative HHD patients, those with UDC and IDC had significantly worse EFS (p=0.01) and overall survival (p=0.03) (Fig. 1b). Finally, we compared the primary and relapse clones in 46 patients. As a result, 37 patients had one or more disease clones at diagnosis, and 29 (78%) had these primary disease clones at relapse. On the other hand, 9 (24%) patients showed relapse-specific disease clones, which should lead to relapse in these cases. The other 9 primary patients with UDC had no disease clone also at relapse.
Discussions: We identified three clonality patterns, UDC, IDC, and CDC based on RNA-seq analysis of the IGH rearrangement status. According to the findings of GSEA and CIBERSORTx, leukemic cells of UDC, IDC, and CDC patients have nature of progenitors to pre-proB cells, pro-B cells, and more mature cells, respectively. These findings are consistent with the knowledge that IGH rearrangement occurs during pro-B to pre-B maturation in normal B-cells. Patients with immature clonality status, UDC and IDC, were associated with poor prognosis in high hyperdiploid subtype and could be a potential prognostic marker in MRD-negative patients. In addition, we revealed that primary disease clones contributed to relapse in 78% of cases, while relapse-specific clones were implicated in relapse in 24% of cases. Additionally, UDC clonality pattern was carried over at relapse. These findings shed light on the mechanisms of leukemic clonal evolution and relapse, and suggest that the status of IGH rearrangement may serve as a novel prognostic marker in BCP-ALL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal